Answer:
Option B
Explanation:
Option (b) shows optical isomerism
[Co(en)3]3+
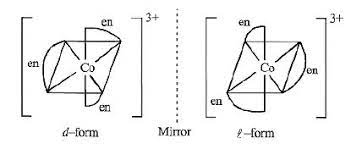
Complexes of Zn++ cannot show optical isomerism as they are tetrahedral complexes with plane of symmetry
[Co(H2O)4(en)]3+have two planes of symmetry hence it is also optically inactive.
[Zn(en)2]2+cannot show optical isomerism